KAPA Express Extract Kits
 Rapid and efficient extraction of DNA from a broad range of source material.
Rapid and efficient extraction of DNA from a broad range of source material.
- Rapid extraction protocol – PCR-ready DNA in 15 minutes
- Versatility – single kit optimized for a variety of sample types
- Single-tube reaction minimizes risk of contamination
- Couple with KAPA2G Robust HotStart ReadyMix for increased PCR success rates
The combination of KAPA Express Extract and KAPA2G Robust HotStart ReadyMix provides the highest performance solution for rapid DNA extraction and consistent downstream amplification. KAPA2G Robust DNA Polymerase is a novel enzyme evolved for increased processivity and exhibits greatly improved tolerance to carryover PCR inhibitors from crude DNA extractions. More information on KAPA2G Robust DNA Polymerase here>>
↓ KAPA Express Extract Brochure
↓ Mammalian DNA barcoding Application Note
↓ Mouse Genotyping Application Note
KK7100 KAPA Express Extract (50 rxn) 100 units of Extract Enzyme (1 U/µL) suitable for 50 DNA extractions. Supplied with 10X Extract Buffer. KK7101 KAPA Express Extract (100 rxn) 200 units of Extract Enzyme (1 U/µL) suitable for 100 DNA extractions. Supplied with 10X Extract Buffer. KK7102 KAPA Express Extract (250 rxn) 500 units of Extract Enzyme (1 U/µL) suitable for 250 DNA extractions. Supplied with 10X Extract Buffer. KK7103 KAPA Express Extract (500 rxn) 1000 units of Extract Enzyme (1 U/µL) suitable for 500 DNA extractions. Supplied with 10X Extract Buffer. KK7151 KAPA Express Extract (100 rxn) and KAPA2G Robust HotStart ReadyMix (100 rxn) 200 units of Extract Enzyme (1 U/µL) suitable for 100 DNA extractions. Supplied with 10X Extract Buffer. Kit also includes 100 x 25 µl reactions of KAPA2G Robust HotStart ReadyMix, a convenient 2X master mix containing KAPA dNTPs, reaction buffer, and Mg2 KK7152 KAPA Express Extract (500 rxn) and KAPA2G Robust HotStart ReadyMix (500 rxn) 1000 units of Extract Enzyme (1 U/µL) suitable for 500 DNA extractions. Supplied with 10X Extract Buffer. Kit also includes 500 x 25 µl reactions of KAPA2G Robust HotStart ReadyMix, a convenient 2X master mix containing KAPA dNTPs, reaction buffer, and Mg Speed: From sample to PCR in less than 15 minutes
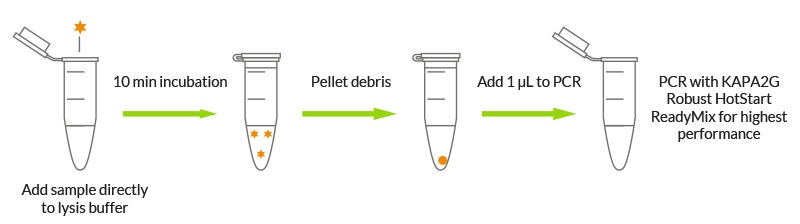
Versatility: Rapid DNA extraction from a variety of sample types
Traditional methods of DNA extraction are time consuming and laborious or require specialized kits optimized for individual tissue types. KAPA Express Extract kits offer a convenient and efficient alternative for the routine extraction of DNA from a variety of tissue types, including buccal swabs, hair follicles, FFPE tissue, bone marrow, blood, blood spots on denim, and processed foods. KAPA Express Extract kits coupled with KAPA2G Robust HotStart ReadyMix (which contains a novel DNA polymerase tolerant of carryover inhibitors), significantly improves PCR success rates when amplifying from crude extracts.
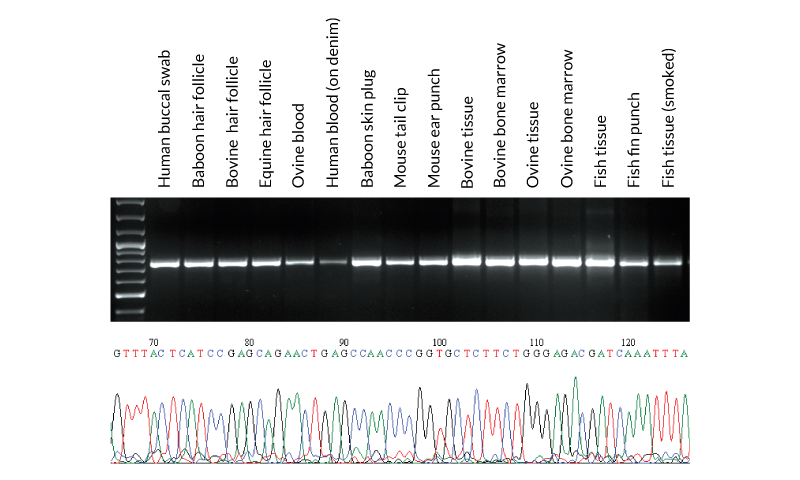
DNA barcoding is rapidly gaining support as a quick, cost-effective and broadly acceptable tool for species identification. DNA was extracted with KAPA Express Extract from various samples obtained from mammals and fish. From each extract, 2 μl was used directly (without quantification) in a PCR containing KAPA2G Robust HotStart ReadyMix and primers for the ~650 bp cytochrome c oxidase I gene fragment commonly used in species identification (Ivanova et al., 2007). PCR products (10 μl) were analyzed in a 1% agarose gel. Sample origin and type is displayed above the gel.
Reaction products were used directly in standard Sanger sequencing reactions using out-nested M13 primers (2 μl PCR product per 10 μl sequencing reaction). Sequence data was of a high quality and enabled the identification of each species. A section of the sequence trace from Seriola lalandi (Yellowtail amberjack) tissue is presented in the bottom panel.
Fast DNA extraction and increased PCR success rates from FFPE tissue
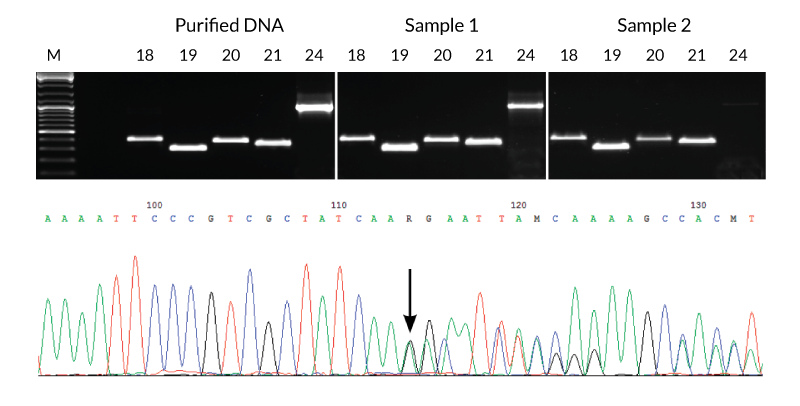
DNA extracts were prepared from two different FFPE samples using KAPA Express Extract. Sample 1 was archived for >6 months and Sample 2 for >1 year. Each extract was used directly (without quantification) in multiple PCRs containing KAPA2G Robust HotStart ReadyMix and primers for five different fragments (293 bp – 1 kb) of the EGFR gene (corresponding to exons 18 – 21 and 24). Results were compared to those obtained using the same reaction and cycling conditions but using 1 ng purified human genomic DNA as template. With the exception of the 1 kb exon 24 fragment from the older sample, yields and reaction efficiencies were comparable between the FFPE DNA extracts and purified genomic DNA. The PCR products generated from sample 1 were diluted 1:10 and used directly in standard Sanger sequencing reactions. Sequence data (bottom panel, Sample 1 exon 19 fragment) was of a high quality. The mixed sequence starting at the position marked with the arrow confirmed the presence of a 15-nt deletion associated with non-small cell carcinoma diagnosed in the patient from whom Sample 1 was collected.
Routine extraction of DNA from a variety of blood sample types
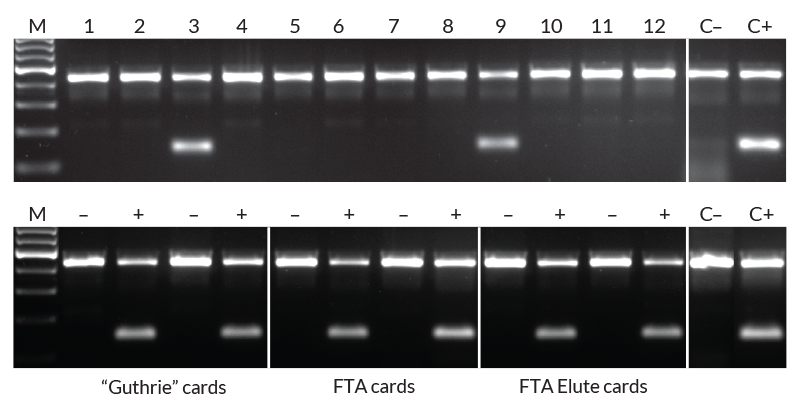
Extraction and amplification of DNA from different blood sample types for detection of the HLA-B*27 allele. DNA was extracted from 12 human EDTA blood samples with KAPA Express Extract (top panel). 2 μl of each extract was added directly to a 25 μl PCR containing KAPA2G Robust HotStart ReadyMix and two primer sets. The internal control primer set targets a 429 bp fragment of the beta globin gene, whereas the second primer set targets a 141 bp fragment of the HLA-B*27 locus in a sequence-specific manner. Two of the 12 individuals tested positive for the HLA-B*27 allele associated with ankylosing spondylitis. Lanes C- and C+ represent HLA-B*27 negative and positive controls respectively (1 ng purified human genomic DNA as template). DNA was extracted from “Guthrie” cards, FTA cards, or FTA Elute cards (bottom panel) spotted with blood of individuals confirmed to be HLA-B*27 positive (+) or negative (-). DNA extraction and amplification conditions and controls (C- and C+) were the same as for the top panel.